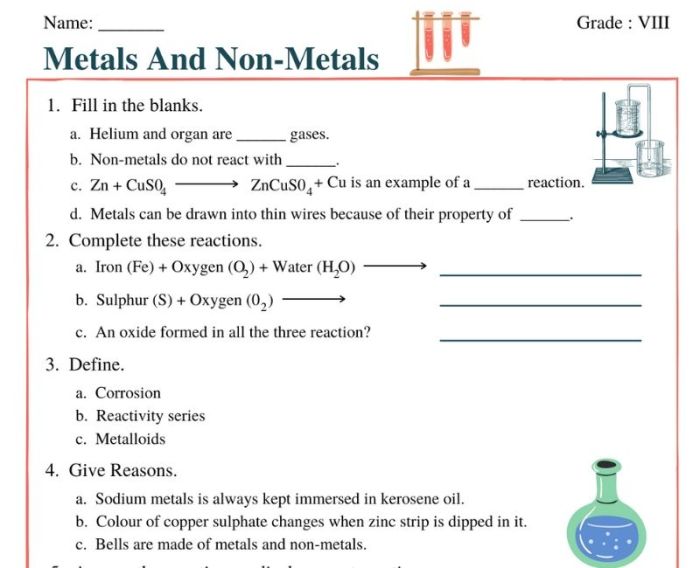
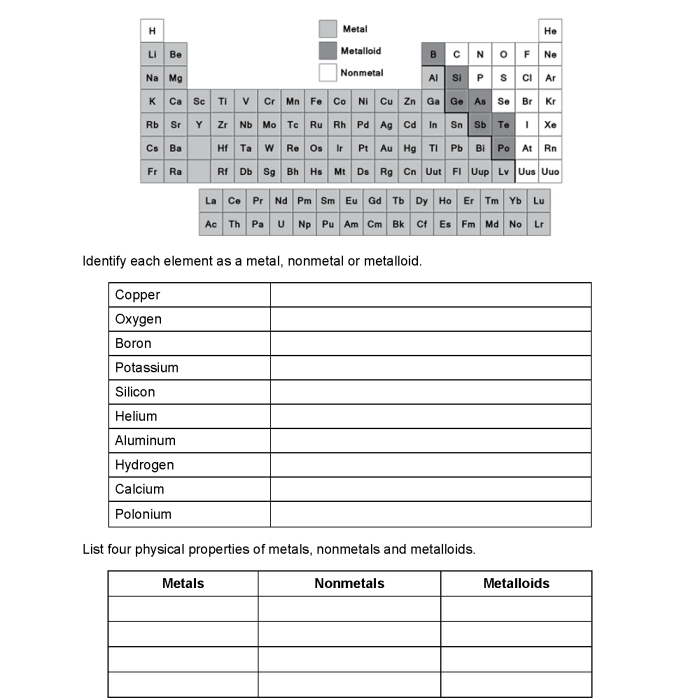
Embark on a scientific journey with our metals nonmetals and metalloids worksheet, an authoritative guide that unravels the fascinating world of these elements and their distinct properties.
Delve into the captivating realm of metals, nonmetals, and metalloids, exploring their unique characteristics and diverse applications in various industries.
Introduction to Metals, Nonmetals, and Metalloids
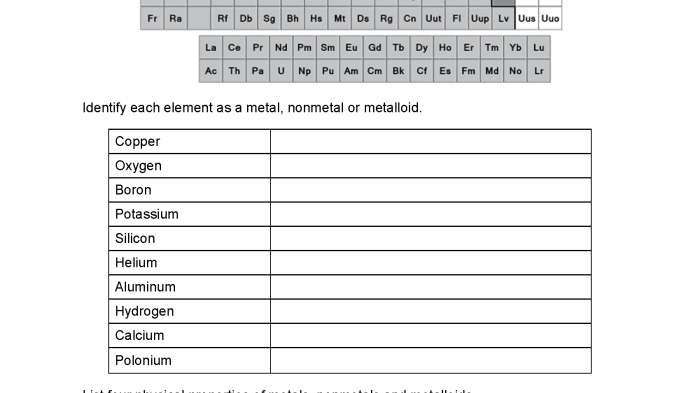
The elements that make up our world can be classified into three broad categories: metals, nonmetals, and metalloids. These groups are distinguished by their physical and chemical properties, which determine their behavior and applications.
Properties of Metals
- Metals are generally shiny and lustrous.
- They are malleable and ductile, meaning they can be easily shaped and drawn into wires.
- Metals are good conductors of heat and electricity.
- They are typically solid at room temperature, except for mercury.
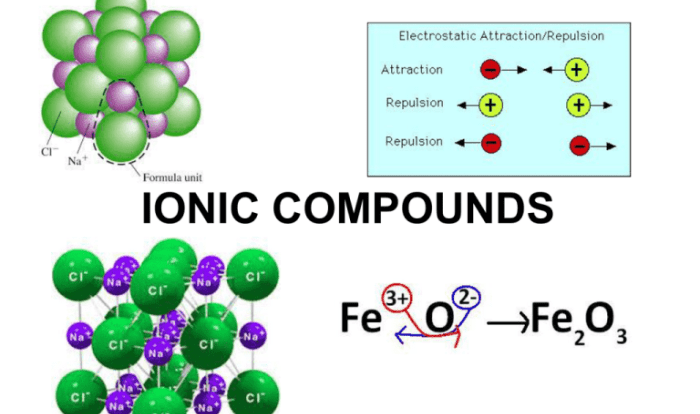
- Metals react with nonmetals to form ionic compounds.
Properties of Nonmetals
- Nonmetals are generally dull and lackluster.
- They are brittle and cannot be easily shaped or drawn into wires.
- Nonmetals are poor conductors of heat and electricity.
- They are typically gases or solids at room temperature.
- Nonmetals react with metals to form covalent compounds.
Properties of Metalloids
- Metalloids exhibit properties of both metals and nonmetals.
- They can be shiny or dull, depending on the specific metalloid.
- Metalloids are semiconductors, meaning they can conduct electricity under certain conditions.
- They are typically solids at room temperature.
- Metalloids can react with both metals and nonmetals to form various types of compounds.
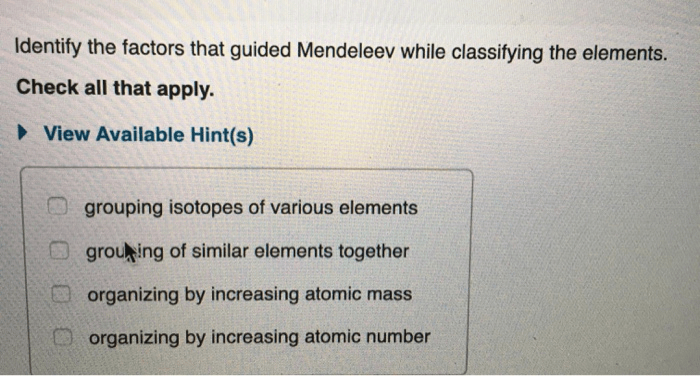
Comparison of Metals, Nonmetals, and Metalloids, Metals nonmetals and metalloids worksheet
| Property | Metals | Nonmetals | Metalloids |
|---|---|---|---|
| Physical Appearance | Shiny, lustrous | Dull, lackluster | Can be either shiny or dull |
| Malleability and Ductility | Malleable and ductile | Brittle | Can be brittle or malleable |
| Electrical Conductivity | Good conductors | Poor conductors | Semiconductors |
| Reactivity | React with nonmetals | React with metals | React with both metals and nonmetals |
Applications of Metals, Nonmetals, and Metalloids
- Metalsare used in a wide variety of applications, including construction, transportation, electronics, and jewelry.
- Nonmetalsare used in a variety of applications, including plastics, fertilizers, and fuels.
- Metalloidsare used in a variety of applications, including semiconductors, solar cells, and thermoelectric devices.
Real-World Examples of Metals, Nonmetals, and Metalloids
- Metals: Iron, copper, aluminum, gold, silver
- Nonmetals: Oxygen, hydrogen, nitrogen, carbon, chlorine
- Metalloids: Silicon, germanium, arsenic, antimony, tellurium
Quick FAQs: Metals Nonmetals And Metalloids Worksheet
What are the key differences between metals, nonmetals, and metalloids?
Metals are malleable, ductile, and good conductors of heat and electricity. Nonmetals are brittle, dull, and poor conductors. Metalloids exhibit properties of both metals and nonmetals.
What are some common examples of metals, nonmetals, and metalloids?
Metals: iron, copper, aluminum. Nonmetals: oxygen, nitrogen, carbon. Metalloids: silicon, germanium, arsenic.
How are metals, nonmetals, and metalloids used in everyday life?
Metals are used in construction, transportation, and electronics. Nonmetals are used in plastics, fertilizers, and fuels. Metalloids are used in semiconductors, solar cells, and optical fibers.